Meet the scientists
18-August-2010
Meet the scientists
Bioengineering
Jon Cooper can remember clearly when he decided to become a scientist, he says. “I was 8 or 9 and we’d got this new science lab at my school on the outskirts of London. You had to walk across the playing fields to get to it. It was like a different world out there. It seemed you were leaving school and going somewhere to have fun.”
Now the leader of a large bioengineering research group, Professor Cooper is clearly still  having fun while doing original research with serious implications. “Science is about curiosity, creativity and imagination,” he says. “You have to try to think differently from other people.”
having fun while doing original research with serious implications. “Science is about curiosity, creativity and imagination,” he says. “You have to try to think differently from other people.”
One area in which researchers are now thinking differently is the diagnosis and treatment of disease. “So traditionally if you’re not feeling well a doctor will take a blood sample and you’ll come back a week later to get the result. What we’re looking at now is point-of-care testing, where you administer your own medicine based on a test you do yourself – and get the result right away.”
The obvious example is the glucose meter, he says. “The person with diabetes is able to respond immediately to the measurement by taking insulin or sugar. So diabetes, which used to kill about 100,000 people a year, can now be managed relatively easily.”
Point-of-care is particularly vital in poor countries, says Professor Cooper, where infectious diseases are rife and healthcare systems struggle. “But it’s also important in poorer areas of developed countries. You get tens of people dying of TB in Glasgow each year, which translates to thousands across the developed world. So this whole idea of doing diagnostics where you don’t need to go to hospital and can get a reading very quickly is increasingly important.”
The challenge is that the devices need to be small and reliable with low power needs, which is a tough set of requirements that will take imaginative engineering to achieve. “We’ve been looking at using sound waves to do the job,” says Professor Cooper. “Surface acoustic waves (SAW) is a technology that’s already used in mobile phones, bank touch screens and TV tuners.
“We’re looking at applying it by putting a drop of fluid in the way of the sound wave instead of your finger. What that gives you is a low cost, low power method of moving your fluid sample, whether it’s blood, urine or saliva.”
But manipulation of the sample is just part of what’s needed. The new devices being developed in Prof Cooper’s group have to analyse and diagnose as well as manipulate – and here too sound waves are the secret, he says.Trypanosome parasites for example are carried in blood and are much larger than red and white blood cells. So they can be detected through their effects on a focused sound wave passing through the sample.
“Malaria is different because the plasmodium that causes it enters the blood cells, so we can’t differentiate by size. But we can by using changes in mechanical properties. Red 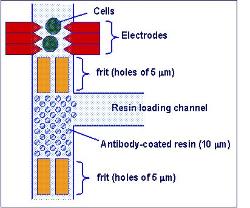 blood cells carrying the parasite are stiffer – which is what causes some of the problems when you have the disease – and again that has an effect on the acoustic wave that we can measure.”
blood cells carrying the parasite are stiffer – which is what causes some of the problems when you have the disease – and again that has an effect on the acoustic wave that we can measure.”
Other ways of engineering fluids with sound being developed in Professor Cooper’s large group of bioengineers include bursting blood cells open – lysing them – to study what’s inside and setting a small droplet spinning like a miniature centrifuge to separate its contents.
“We’ve also shown that we can make devices with a material that you can throw away after the test and replace. This very much reduces the cost. It’s a similar model to a disposable razor where you have a nice head and handle, but blades are used and thrown away. It enables you to make cheap, practical devices
All this is just one area of research in Professor Cooper’s bioengineering group, he says. So what exactly is bioengineering? “That’s the interface between engineering – electronics, mechanics, materials – and biology,” says Professor Cooper. “It’s what we do.”
More on Professor Jon Cooper's research, together with lesson plans.
More help with words
| application | average | bacteria | cell | complex | density |
| engineered | insulin | membrane | mass | molecule | pancreas |
| protoplasm | protein | structure | symptom | tissue | volume |
Molecular parasitology

Anyone who names Paul Cézanne as his favourite scientist, has to be a little confused, you might think. The post-impressionist was a master of design, colour and composition, but he wasn’t a scientist. “Maybe not,” says Professor Dave Barry. “But he was taking new knowledge in physics and applying it to painting. Scientists often say that science and art are similar. But a scientist is doing it up here while the painter is doing it here and here.”
He touches his head and heart. “It’s digging deeper. I just wish, as a scientist, I could learn to harness both those sources in me. It’s nothing to do with mysticism. It’s about knowledge and the creativity that comes from knowledge.
“Because of the way we’re trained, scientists all tend to think the same way. There are other ways. I’ve been able to tap into those only rarely, when painting myself, So for me these great painters, and particularly Cézanne, were scientists as well as artists.”
Collaborations between science and art should therefore be able to produced something new and exciting in science, Professor Barry believes. But they rarely have done. “There have been some quite good projects but no real synergy. I tried it myself. I wanted to tap into deep scientific principles and phenomena, but I was going too deeply for the artist, while his approaches to painting were beyond me.”
So until the right artist comes along Professor Barry and his group – as well as the Wellcome Trust Centre for molecular parasitology, of which he is director – will continue to work in purely scientific ways, although with increasingly sophisticated methods and technologies.
Collaboration is still the key. “Here at the Centre we have the biggest concentration of parasitologists in Europe. We try to understand how parasites work, spread and interact with their hosts – and to use that knowledge to develop drugs, vaccines and diagnostics.”
The malaria parasite and trypanosomes are the main areas of research interest, he says. “These are responsible for much of the devastation in Africa. So my group is looking at the very clever ways the parasites evade immunity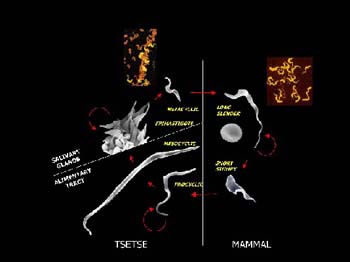 in their hosts. We now know that trypanosomes have 2000 genes for making different coats, and that they can combine these genes in an almost endless variety of ways. Antibodies can destroy those with one particular coat, but a small number of parasites in the population will have different coats. They’ll then multiply and the immune system has to start all over again.”
in their hosts. We now know that trypanosomes have 2000 genes for making different coats, and that they can combine these genes in an almost endless variety of ways. Antibodies can destroy those with one particular coat, but a small number of parasites in the population will have different coats. They’ll then multiply and the immune system has to start all over again.”
It sounds like a hopeless task, but advances in genetics are creating new possibilities, he says. “We might for instance develop drugs that attack the biochemical processes that change the parasite’s coats – and because that’s quite different to the host, the drug can be specific and so avoid the side-effects drugs have when they hit similar processes in host cells.”
One of the most promising new approaches arises from collaboration with ecologists and mathematicians, Professor Barry believes. Initial doubt is rapidly giving way to excitement at the possibilities. “It has been an eye-opener for us looking at transmission, re-infection, how the parasite behaves in the field and interacts with the host. I never thought we’d be able to ask some of these questions, never mind answer them.
“As one of my mathematical colleagues says – it doesn’t matter if it’s a population of elephants on the plains or a population of molecules in a cell. We can model them. These are very powerful new methods.”
More on Professor Dave Barry's research, together with lesson plans.
More help with words
| antibodies | atom | cell | chemistry | conception |
| DNA | element | fertilisation | fundamental | gene |
| host | immunity | Impressionism | inherit | laboratory |
| membrane | micro-organism | molecule | parasite | particle |
| process | protein | protoplasm | structure | subtle |
Molecular and cellular biology
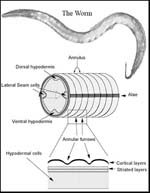
The reason we are not all shapeless blobs is connective tissue, says Iain Johnstone. “That’s what gives our bodies shape and structure. Every organ in our bodies is held together by connective tissue. Without it we would just be a soup of cells.
“Now we know how connective tissue can hold cells together. But how does it hold one of the chambers of your heart or the complex structures in a kidney in exactly the right shape to do their job? That is the big question.
“It’s much more than just a glue. It’s the way it glues cells in a coordinated way. The term is morphology. So how do tissues end up with the correct morphology? There is a huge gap of knowledge there.”
Morphology is not just hard for the scientists it is, on the face of it, extremely difficult for the developing organism. These building blocks of connective tissue – these huge molecules secreted by cells but existing outside them – have to assemble themselves, says Dr Johnston. “An engineer building a bridge will work out how to put the building blocks together.
“He’ll step back and look at the structure taking shape. But these things have to assemble without conscious input from a mind. The molecular machinery can’t hold itself up to a mirror and check it’s got the shape correct. It just does it. Shape generation is incredible.”
Our bones for instance have huge amounts of connective tissue, he says, and many of the more severe connective tissue diseases are bone defects. “The most crippling is osteogenesis imperfecta – brittle bone disease – in which people have multiple fractures, reduced stature and twisted skeletons.”
Much of the research in Dr Johnston’s group is on connective tissue proteins called collagens. The organism in which they study these is a little worm called Caenorhabditis elegans – C. elegans for short. “It’s about one millimetre long, and is a simple model system to study many processes. Most animals have many of the same key molecular mechanisms, and the worm has a lifecycle of two and a half days, so you can do a lot of work very quickly.”
Understanding a complex system like the manufacture and assembling of collagen is a bit like understanding a motor car, says Dr Johnston. “You need to know what the separate parts do before you understand the whole system.”
The component parts of a motor car are pistons, valves, camshafts etc. In a biological system they are genes and the proteins they encode. The connective tissue assembled from these proteins corresponds to the car – except that its structure is more complex than any car, or indeed any machine built by humans.
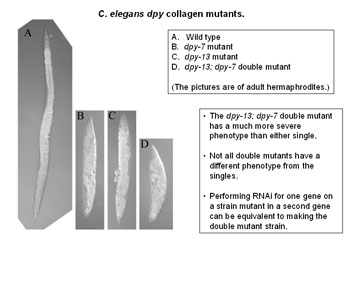
A molecular biologist uses a variety of techniques for studying the parts. Knocking out a particular gene and seeing what happens is one of the most common, says Dr Johnston. “Another is immuno-fluorescence detection, which lets us see the shape of a structure.”
Using these methods Dr Johnstone’s team have discovered the precise effect of a particular gene called DPY-7 – pronounced “dumpy 7” – on the little worm C. elegans. Knocking out this gene – whether it is done artificially in the lab or by natural mutation – means that bands of tissue, like corsets around the worm’s body, fail to develop and it grows short, fat and, in a word, dumpy.
Exactly the same type of mutation causes diseases such as osteogenesis imperfecta in humans. But there is one major difference – a difference that fascinates Dr Johnstone and offers, he believes, the possibility of a cure for these terrible diseases. “In humans the mutations are dominant, but in C. elegans they are predominantly recessive.
“The defect is the same. But the worm has some capacity humans lack to allow the wild type copies to go out and do their job. Now that is very interesting indeed. I can’t prove yet that understanding how the worm does it will lead to a cure in humans. But it is certainly worth a shot.”
More on Dr Iain Johnstone's research, together with lesson plans.
More help with words
| antibody | cell | complex | conception | DNA | expressed |
| fertilisation | fluorescent | identical | inherit | membrane | molecule |
| protein | protoplasm | radiation | technique | ultraviolet | wavelength |

