Introduction
Plastid organelles trace their evolutionary origins to cyanobacteria that were incorporated into eukaryotic cells by a process of endosymbiosis. This evolutionary history dictates that they cannot be formed de novo. Instead, existing plastids divide to give rise to daughter organelles that partition into daughter cells upon cell division. Previously studied plastids contain an FtsZ-based division apparatus retained from the cyanobacterial endosymbiont [1]. In addition, plant and red algal plastid division involves a dynamin-like protein called ARC5 (also known as DRP5B [2] and [3] ).
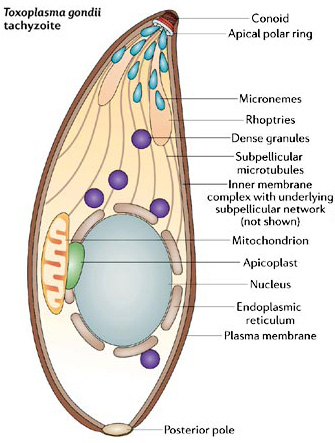
Apicoplasts, the nonphotosynthetic plastids of apicomplexan parasites, must correctly divide and segregate into daughter cells for parasites to remain viable [4]. Surprisingly, apicomplexan genomes lack homologs to both ARC5 and FtsZ [5], suggesting that apicoplast division is mechanistically different than that in previously studied plastids. One striking difference is the association of the apicoplast with the centrosomes of the mitotic spindle [6] and [7] . This association is thought to ensure proper segregation during cytokinesis, parceling out apicoplasts to a highly variable number of daughter cells formed in the complex apicomplexan budding process [8]. Though centrosome association provides a unifying model for segregation, it remains unclear how apicoplast fission occurs. One model suggests that fission depends on force generated by daughter cell budding [6], whereas electron microscopic studies identify apparent plastid division rings [9] and [10] , suggesting that protein components may mediate fission.
Dynamins are large GTPase proteins that function in a range of contractile processes, including the scission of endocytic vesicles, cytokinesis, nuclear remodelling, and the fission of mitochondria, chloroplasts, and peroxisome organelles [11] , [12] and [13] , and we were interested in whether dynamins had a role in apicoplast division. Apicomplexan genomes encode three dynamin-related proteins that are phylogenetically distinct from ARC5. In this study, we characterize dynamin-related protein A (DrpA) in the apicomplexan T. gondii. We demonstrate that TgDrpA is required for apicoplast fission, and we present a detailed model for how TgDrpA functions in this process.