science
Please mouseover difficult words to see what they mean
News
Stanford, CA: 14-Dec-2007 14:00 Eastern US Time
Coral reefs in acid oceans
Carbon emissions from human activities are not just heating up the world. They are changing the ocean’s chemistry. This could soon be fatal to coral reefs.
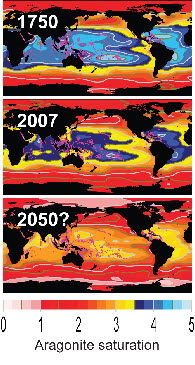
These reefs are havens for biodiversity in the sea. Many coastal communities make a living from them. But if carbon dioxide emissions continue to rise, then by the middle of this century 98% of reef habitats will be bathed in water too acid for the reef to grow.
This is the main conclusion of calculations by scientists at the Carnegie Institution’s Department of Global Ecology
Among the first to feel the effects will be Australia’s Great Barrier Reef, the world’s largest living thing.
Chemical oceanographers Ken Caldeira and Long Cao report their results in a multi-author paper in the December 14 issue of Science. They also present them at the annual meeting of the American Geophysical Union in San Francisco on the same date.
The work is based on computer simulations of ocean chemistry. The scientists looked at the effects of different levels of carbon dioxide in the atmosphere. The levels ranged from 280 parts per million to 5000 parts per million.
The first is the level before there were any industries in the world. The second is much higher than the present level. That is roughly 380 ppm.
Carbon dioxide levels in the atmosphere are rising fast because of increasing emissions from human activities. The main one is the burning of fossil fuels.
“About a third of the carbon dioxide put into the atmosphere is absorbed by the oceans,” says Caldeira. "This helps slow greenhouse warming. But it is a major pollutant of the oceans.”
The carbon dioxide absorbed in the sea produces carbonic acid. This is the acid in soft drinks that gives them their fizz. What it does in the ocean is make certain minerals dissolve more easily in seawater.
This is especially true for aragonite. This is the mineral used by corals and many other marine organisms to grow their skeletons.
Before the industrial revolution, almost all warm-water coral reefs were bathed with open ocean waters which was 3.5 times supersaturated with aragonite, says Cao. "This means that corals could easily extract it to build reefs.”
But if atmospheric carbon dioxide sticks at 550 ppm, no existing coral reef will remain in such an environment. The chemical changes will affect some regions sooner than others. At greatest risk are the Great Barrier reef and the Caribbean Sea.
Levels of carbon dioxide are rising rapidly, Cao added, and even to get them to stick at 550 ppm would take concerted international efforts. These look very unlikely at the moment.
Carbon dioxide’s chemical effects on the ocean are pretty much independent of its effects on climate. So efforts to slow global warming won't slow the acidification of the seas, the researchers say. That is unless emissions are reduced.
In fact, the expected chemical changes in the sea may need emissions to be cut even more than for climate alone.
“These changes come at a time when reefs are already stressed by climate change, overfishing, and other types of pollution,” says Caldeira. “So unless we take action soon there is a very real possibility that coral reefs - and everything that depends on them - will not survive this century.”
Hyperlink highlights
Don’t miss the supersaturated video from PBS. It’s cool, instructive and very entertaining.
More help with words
average
conference
dissolved
elements
fossilised
greenhouse gas
journal
models
solution
variety
What's it all about?
Topics for discussion, research or pupil presentations
Here some of the issues, implications and applications taken directly from the text. Students should be encouraged to find others themselves.
Here are some possible topics for group discussion, research or presentations arising from these. Students should be encouraged to think up others: